Inspection systems for COVID-19 test kits
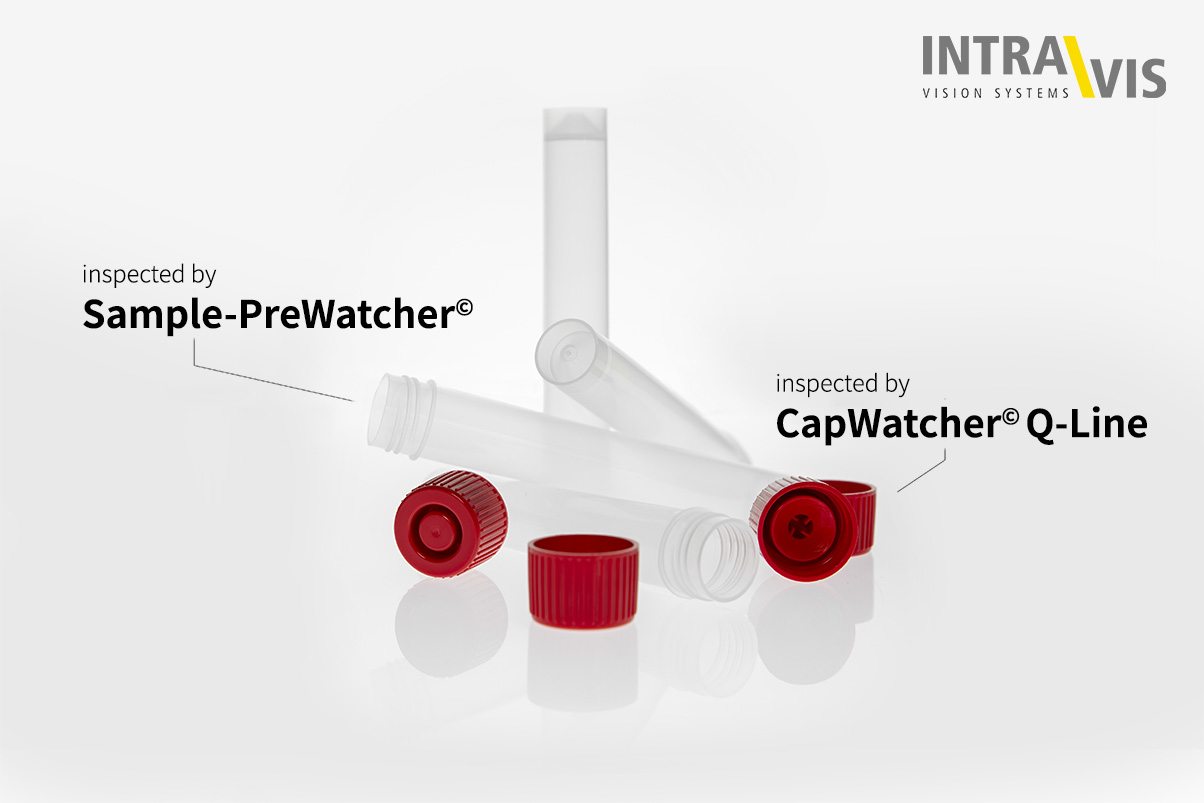
We now support a manufacturer of COVID-19 test kits with two of our solutions for vision inspection. The inspection systems Sample-PreWatcher and CapWatcher Q-Line are used for the inspection of tubes and caps of the test kits. Our years of experience in the pharmaceutical industry played a major role in the successful implementation of this important and urgent project.
"We are pleased and proud to be able to contribute to the containment of this virus," says Dr. Gerd Fuhrmann, CEO of Intravis GmbH, happily about the cooperation with the renowned manufacturer of the COVID-19 test kits. The kits consist of a tube and a cap. While a Sample-PreWatcher is used for the tube, the cap is inspected by a CapWatcher Q-Line.
The systems are equipped for typical applications in the pharmaceutical sector as well as in the food and cosmetics sector. The COVID-19 test kits are analyzed for various characteristics so that each test can be evaluated in the laboratory without external influences. It is particularly important that the systems detect all forms of contaminations as well as possible damages of the individual test kit components and sort out the corresponding parts.
The current pandemic situation makes it necessary that the shortest possible delivery time had to be guaranteed. Thus, only 12 weeks were required between receipt of the order and commissioning of the systems at the customer's site. Helpful for a quick implementation of this critical project was also Intravis' years of experience in the pharmaceutical industry. Dr. Fuhrmann says "We count some of the world's largest manufacturers of pharmaceutical packaging among our long-standing customers. In the projects with these customers, we have gained important experience over the years regarding regulations in the pharmaceutical industry. Of course, this has now been of benefit for us."
Intravis also confirms that other manufacturers of test kits have already expressed interest in comparable inspection systems and that the company considers itself well positioned for further inquiries.